Blood Vessel Growth in the Zebrafish Fin
Quantitative Analysis of Sprouting Angiogenesis in the Zebrafish Fin
Jennifer Lanni, William Gan ‘21 (Biology), and Jeanne Specht ‘23 (Biochemistry and Hispanic Studies)
Wheaton College, Norton, MA
The main focus of our research was to quantitate a physiological process known as angiogenesis. During angiogenesis, new blood vessels are developed from previously existing ones, and they sprout, or split apart, from those pre-existing vessels. This allows oxygen and vital nutrients to reach different tissues in the body, promoting both development and growth. We need this process in order to heal wounds and to regenerate injured tissues. However, abnormal blood vessel growth is a major underlying cause of many conditions, such as cardiovascular disease, cancer, and more (Grisham 2014). By studying angiogenesis, we can observe how different factors may affect blood vessel growth and consider potential treatments that could inhibit or stimulate angiogenesis, preventing future disease. In order to do so, we decided to study the process of sprouting angiogenesis within zebrafish.
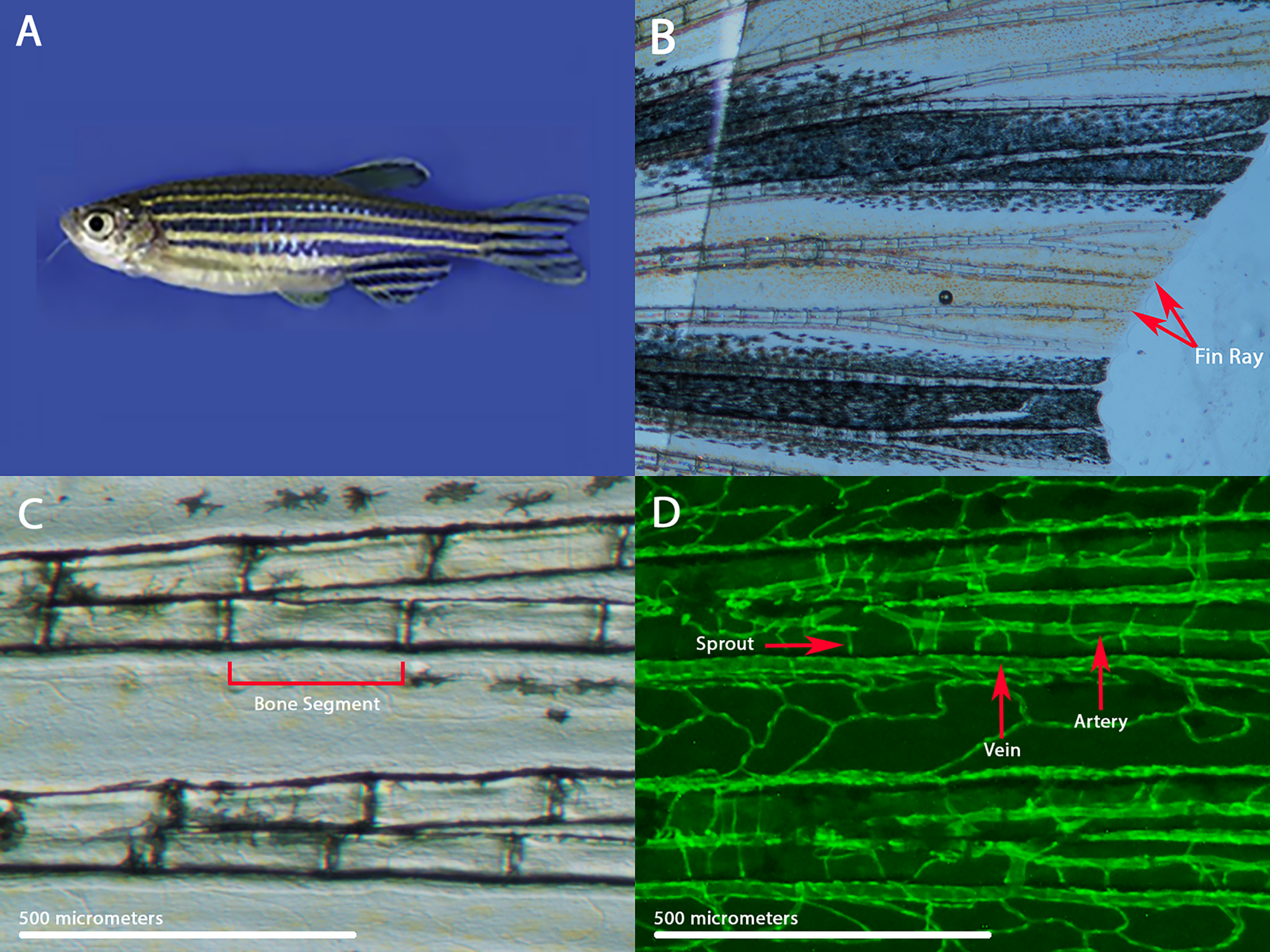
Zebrafish are small freshwater fish that are very useful in biomedical research. They have the same organs and tissues as larger vertebrates, and they share many of the same genes. Zebrafish can also be genetically modified to study specific genes or to make it easier to visualize a biological process. The fins of zebrafish contain long bony rays that are made of individual bone segments. Each fin ray contains an artery that brings blood to the fin tip and two veins that bring blood back to the body. These blood vessels can easily be seen under the microscope and analyzed throughout their life cycle.
During normal fin development, the blood vessels grow by vascular branching morphogenesis, in which sprouts emerge and connect each artery to its flanking veins (intraray vessels). Fin rays that are injured can regenerate their blood vessels along with any injured or missing tissue. Different mutations and pharmacological treatments can affect the pattern of the fin blood vessels, including the density of intraray vessels (Huang 2003). Our goal was to develop a methodology to quantitate and compare the sprouting angiogenesis present in zebrafish. To investigate this phenomenon, we observed and collected data in transgenic zebrafish marked with green fluorescence protein that labeled the arteries and blood vessels present in the caudal fin rays.
Both of us found the experience of performing research as remote students to be uniquely challenging but still rewarding. For Jeanne, the greatest difficulty during this project was designing an entirely new procedure for quantitative data analysis while also participating remotely and in a different time zone. “Although it was challenging at times, I wouldn’t trade this experience for anything because it made me a better teammate and a stronger researcher. Now that I’m back on campus and conducting research in the lab, I’m even more excited about all of the discoveries we’re going to make!” Will found it difficult but rewarding to learn about a new biological process while simultaneously developing the tools to quantitate it. “Participating in research during a global pandemic presented many challenges. Despite our initial research being remote, it allowed us to explore various techniques to understand our data outside of the lab. I strongly believe that this experience has given me a new perspective on biological research and has pushed me to new heights. I am thrilled to be back in the lab this semester and diving into the benchwork!”
References:
Grisham, Julie (2014, March 13). What Is Angiogenesis? Memorial Sloan Kettering Cancer Center. https://www.mskcc.org/news/what-angiogenesis.
Huang, C. C., Lawson, N. D., Weinstein, B. M., & Johnson, S. L. (2003). reg6 is required for branching morphogenesis during blood vessel regeneration in zebrafish caudal fins. Developmental Biology, 264(1), 263–274. https://doi.org/10.1016/j.ydbio.2003.08.016
-
Categories:
- Academic Festival
- Biochemistry
- Biology